A groundbreaking study in mice opens new doors in mitochondrial medicine—and offers clues for enhancing energy, resilience, and longevity at the cellular level
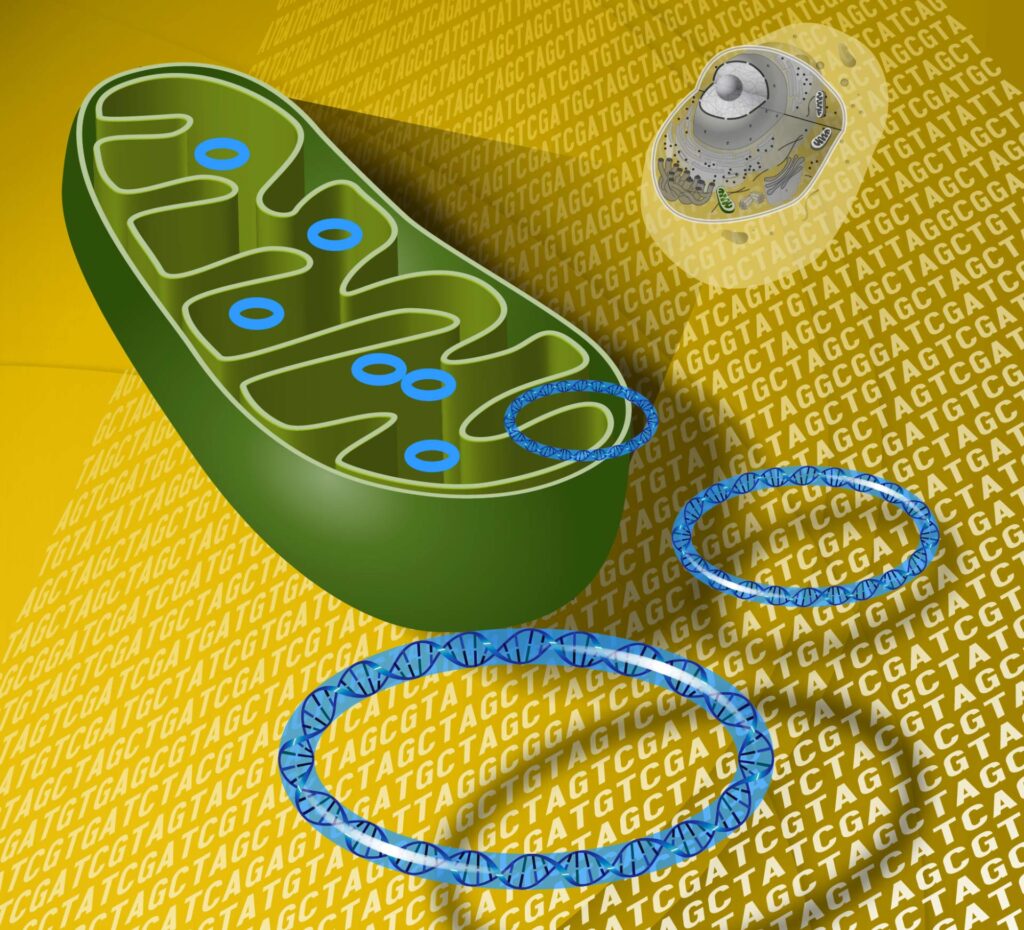
Deep within every cell in your body, two genomes coexist. One resides in the nucleus, home to over 99% of your genetic material. The other—a tiny, powerful remnant of ancient bacteria—lives in the mitochondria, your cells’ energy factories. This second genome is tiny but mighty, containing the instructions for building core components of the energy machinery that powers life itself.
But there’s a catch: mitochondrial DNA (mtDNA) is vulnerable. Unlike nuclear DNA, it lacks protective histones and has limited repair capacity. Over time, and particularly with age, this leads to mutations and dysfunction—one of the central causes of age-related decline.
Now, a new study has explored an ambitious idea: what if we could transfer a critical mitochondrial gene into the nucleus, where it would be better protected? The results, conducted in mice, not only show that this is biologically possible—they suggest that it could one day lead to more robust cells, better aging, and new treatments for mitochondrial diseases.
Let’s explore what this breakthrough means, how the experiment worked, and what it could mean for the future of human vitality.
The Mitochondrial Challenge: Energy, Aging, and Genetic Fragility
Mitochondria are often called the “powerhouses” of the cell because they convert nutrients into ATP, the energy currency your body uses for everything from thinking to movement. But they’re also involved in:
- Regulating cell death (apoptosis)
- Managing calcium levels
- Generating signaling molecules like ROS (reactive oxygen species)
- Supporting immune function
To carry out these functions, mitochondria rely on a dual genetic system:
- Nuclear DNA (nDNA) encodes the vast majority of mitochondrial proteins.
- Mitochondrial DNA (mtDNA) encodes just 13 proteins—but they are absolutely essential for the mitochondrial respiratory chain, which produces ATP.
Unfortunately, mtDNA is highly susceptible to damage:
- It is exposed to free radicals generated during energy production.
- It lacks robust DNA repair mechanisms.
- It is inherited maternally and doesn’t benefit from recombination (genetic reshuffling).
With age, mutations in mtDNA accumulate, contributing to a host of problems: reduced energy, increased oxidative stress, and chronic inflammation. These issues have been implicated in conditions from Alzheimer’s and Parkinson’s to sarcopenia and cardiovascular disease.
So scientists have long wondered: Could we shield mitochondrial genes by expressing them in the nucleus instead?
The Study: Moving a Mitochondrial Gene to the Nucleus in Mice
In a landmark experiment, researchers genetically engineered mice to express a mitochondrial gene—ND1 (NADH-ubiquinone oxidoreductase chain 1)—from within the nuclear genome.
ND1 is normally encoded only by mtDNA and is a critical component of Complex I in the mitochondrial respiratory chain. Without it, cells can’t efficiently generate ATP.
What they did:
- Researchers designed a version of the ND1 gene that included a mitochondrial targeting sequence (MTS)—a kind of address label that tells the cell to send the protein into the mitochondria.
- This gene was inserted into the nuclear DNA of mouse embryos using established genetic engineering techniques.
- They verified that the nuclear-encoded ND1 protein was successfully produced in the cytoplasm, imported into mitochondria, and functionally incorporated into Complex I.
What they found:
- The nuclear version of ND1 was correctly processed and assembled in mitochondria.
- Mitochondrial respiratory function was restored in cells lacking native ND1, confirming that the gene was functionally active.
- There were no adverse effects in the mice—suggesting that the engineered gene was biocompatible and did not interfere with normal cellular operations.
In essence, the researchers had achieved a synthetic rescue—using the nucleus to bypass potential mtDNA damage and restore mitochondrial energy production.
Why This Is a Big Deal
This achievement is more than just a clever genetic trick—it represents a fundamental shift in how we might approach mitochondrial dysfunction and aging.
1. Proof of Concept for “Allotopic Expression”
The process of expressing a mitochondrial gene in the nucleus is called allotopic expression. It’s been tried in cell cultures and yeast, but this is one of the first successful demonstrations in a live mammal.
This lays the groundwork for future gene therapies targeting mitochondrial disorders, many of which are currently untreatable.
2. A Potential Longevity Lever
Since mitochondrial dysfunction is a hallmark of aging, the ability to “rescue” or stabilize mitochondrial genes may extend cellular function and delay age-related decline.
Imagine a therapy that could “back up” your critical mitochondrial genes into the nucleus—safeguarding them from damage and maintaining energy levels deep into later life.
3. Better Understanding of Mitochondrial Evolution
This study also lends insight into evolution. Mitochondria originated from free-living bacteria that entered a symbiotic relationship with early eukaryotic cells. Over time, most of their genes migrated to the nucleus—but a few remained.
Understanding why some genes (like ND1) stayed behind, and whether they can be safely relocated, deepens our grasp of cellular biology.
Challenges Ahead: Why This Isn’t Yet a Human Therapy
Despite its promise, allotopic expression remains technically complex and raises important questions.
Targeting Accuracy
Even with MTS signals, not all nuclear-encoded mitochondrial proteins reach their destination efficiently. Precision targeting is essential for proper mitochondrial assembly.
Stoichiometric Balance
Mitochondrial protein complexes require precise ratios of subunits. Overexpressing one protein—even a functional one—can throw off the balance and impair energy production.
Safety and Long-Term Effects
So far, no harmful effects were seen in the mice. But human trials would require long-term studies to rule out subtle imbalances or immune responses.
Still, this study provides a critical foundation for moving forward with therapeutic development.
Mitochondria and You: Supporting Your Energy Engine Naturally
While gene therapy may be years away, this study reinforces what we already know: mitochondrial health is essential for thriving at every age. Here’s how you can support it naturally:
1. Feed the Mitochondria
Include nutrients that support mitochondrial function:
- CoQ10 for electron transport
- Alpha-lipoic acid for antioxidant protection
- Magnesium for ATP synthesis
- B vitamins (especially B2, B3, and B12) as cofactors in energy production
2. Stimulate Mitochondrial Biogenesis
- Exercise, particularly high-intensity interval training (HIIT), triggers new mitochondria formation.
- Cold exposure and heat therapy (saunas) may activate mitochondrial stress pathways that build resilience.
3. Support NAD+ Levels
NAD+ is essential for mitochondrial enzymes. Consider:
- NMN or NR supplementation
- Intermittent fasting or time-restricted eating
- Good sleep, which helps restore NAD+ via circadian alignment
4. Reduce Mitochondrial Toxins
- Minimize processed foods, environmental toxins, and chronic stress
- Avoid unnecessary exposure to pesticides, industrial pollutants, and excessive alcohol
These strategies help preserve the mitochondria you already have—and keep your energy system humming as long as possible.
Final Thoughts: Future-Proofing the Powerhouse
This research may sound futuristic, but its message is grounded in something simple: mitochondria matter. They are the engines of life, and as they go, so go we.
By showing that it’s possible to move and protect essential mitochondrial genes within the nucleus, this study opens the door to a new class of interventions—ones that aim not to mask aging symptoms, but to restore cellular integrity at the source.
For those invested in wellness, this is a powerful reminder: the health of your mitochondria shapes everything—how clearly you think, how deeply you sleep, how resilient you feel, and how long you live with vitality.
And thanks to advances like these, we may soon have even more ways to safeguard those tiny engines—for decades to come.
